Classification of
Solvents
There are two broad
solvent categories, and they are not mutually exclusive; that is, a solvent can
be in more than one category.
1. A solvent can be
protic or aprotic.
2. A solvent can be
polar or apolar.
1. Protic or Aprotic
Solvent.
A protic solvent consists
of molecules that can act as hydrogen-bond donors.
Water, alcohols, and
carboxylic acids are examples of protic solvents.
Solvents that cannot
act as hydrogen-bond donors are called aprotic solvents.
Ether, methylene chloride,
and hexane are examples of aprotic solvents.
2. Polar or Apolar
(Nonpolar )Solvent.
A polar solvent has
a high dielectric constant;
An Apolar solvent
has a low dielectric constant.
Dielectric
constant
The dielectric
constant is defined by the electrostatic law, which gives the interaction
energy E between two ions with respective charges q1 and q2 separated by a
distance r:
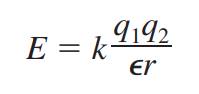
In this equation, k is
a proportionality constant and ε is
the dielectric constant of the solvent in which the two ions are imbedded. This
equation shows that when the dielectric constant ε is
large, the magnitude of E, the energy of interaction between the ions, is
small. This means that
both attractions
between ions of opposite charge and repulsions between ions of like charge are
weak in a polar solvent.
Thus, a polar solvent
effectively separates, or shields, ions from one another. This means, in turn,
that the tendency of oppositely charged ions to associate is less in a polar
solvent than it is in an apolar solvent.
If a solvent has a
dielectric constant of about 15 or greater, it is considered to be polar. Water
(ε =
78), methanol (ε =
33), and formic acid (ε =
59) are polar solvents.
Hexane (ε =
2), ether (ε =
4), and acetic acid (ε =
6) are apolar solvents
Unfortunately, the
word polar has a double usage in organic chemistry. When we say that a molecule
is polar, we mean that it has a significant dipole moment, μ . When
we say that a solvent is polar, we mean that it has a high dielectric
constant.
In other words, solvent
polarity, or dielectric constant, is a property of many molecules acting
together, but molecular polarity, or dipole moment, is a property of individual
molecules.
Although it is true
that all polar solvents consist of polar molecules, the converse is not
true.
The contrast between
acetic acid and formic acid is particularly striking:
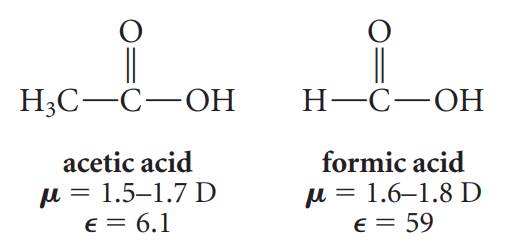
These two compounds
contain identical functional groups and have very similar structures and dipole
moments. Both are polar molecules. Yet they differ substantially in their
dielectric constants and in their solvent properties! Formic acid is a polar
solvent; acetic acid is not.