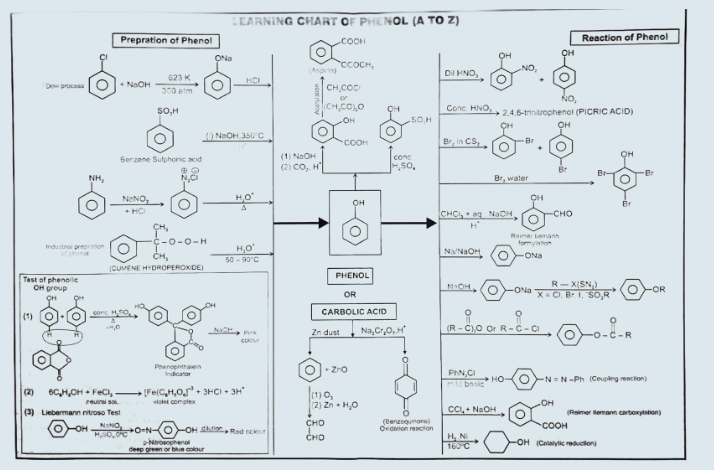
Phenol
Preparation
1. From
haloarenes
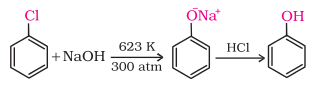
Chlorobenzene
is fused with NaOH at 623K and 320 atmospheric pressure. Phenol is obtained by
acidification of sodium phenoxide so produced.(SNAr)
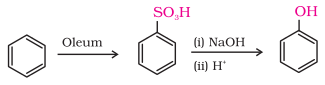
2. From
benzenesulphonic acid
Benzene is
sulphonated with oleum and benzene sulphonic acid so formed is converted to
sodium phenoxide on heating with molten sodium hydroxide. Acidification of the
sodium salt gives phenol. (SNAr)
3. From
diazonium salts
A
diazonium salt is formed by treating an aromatic primary amine with nitrous
acid (NaNO2 + HCl) at 273-278 K. Diazonium salts are hydrolysed
to phenols by warming with water or by treating with dilute acids. (SNAr)

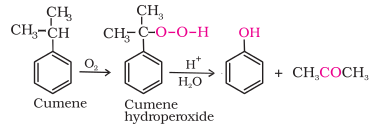
4. From
cumene
Phenol
is manufactured from the hydrocarbon, cumene. Cumene (isopropylbenzene) is
oxidised in the presence of air to cumene hydroperoxide. It is converted to phenol
and acetone by treating it with dilute acid. Acetone, a by-product of this
reaction, is also obtained in large quantities by this method.
5. From Coal Tar:
A number of phenols are present in coal tar, from
which they may be separated by extraction with alkali. Acidification releases
the phenols. Phenol itself, and o-, m-, and p-methylphenols (o-, m-, and
p-cresols) may be obtained in this way.
Reactions
of phenols
1. Acidity of phenols
(i)
Reaction with metals: phenols react with active metals such as sodium,
potassium and aluminium to yield phenoxides and hydrogen.

In addition to this, phenols react with aqueous sodium
hydroxide to form sodium phenoxides.
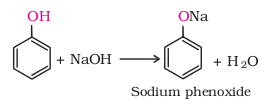
The above reactions show
that phenols are acidic in nature.
(ii) Acidity of phenols: The reactions of phenol with
metals (e.g., sodium, aluminium) and sodium hydroxide indicate its acidic nature.
The hydroxyl group, in phenol is directly attached to the sp2 hybridised
carbon of benzene ring which acts as an electron withdrawing group. Due to
this, the charge distribution in phenol molecule, as depicted in its resonance
structures, causes the oxygen of –OH group to be positive.
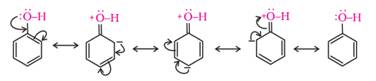
The reaction of phenol with aqueous sodium hydroxide
indicates that phenols are stronger acids than alcohols and water.
In substituted phenols, the presence of electron
withdrawing groups such as nitro group, enhances the acidic strength of phenol.
This effect is more pronounced when such a group is present at ortho and para
positions. It is due to the effective delocalisation of negative charge in
phenoxide ion.
On the other hand, electron releasing groups, such as
alkyl groups, in general, do not favour the formation of phenoxide ion
resulting in decrease in acid strength. Cresols, for example, are less acidic
than phenol.
COMPOUND
|
FORMULA
|
PKA
|
o-Nitrophenol
|
o-O2N-C6H4-OH
|
7.2
|
m-Nitrophenol
|
o-O2N-C6H4-OH
|
8.3
|
Phenol
|
C6H5-oH
|
10.0
|
o-Cresol
|
o-CH3-C6H4- OH
|
10.2
|
m-Cresol
|
m-CH3C6H4-OH
|
10.1
|
p-Cresol
|
p-CH3-C6H4-OH
|
10.2
|
Ethanol
|
C2H5OH
|
15.9
|
pKa Values of Phenols and Ethanol
|
||
2. Esterification
Phenols react
with carboxylic acids, acid chlorides and acid anhydrides to form esters.

The reaction with carboxylic acid and acid anhydride
is carried out in the presence of a small amount of concentrated sulphuric
acid. The reaction is reversible, and therefore, water is removed as soon as it
is formed.
The reaction with acid chloride is carried out in the
presence of a base (pyridine) so as to neutralise HCl which is formed during
the reaction. It shifts the equilibrium to the right hand side.
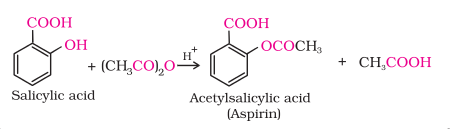
The introduction of acetyl (CH3CO) group
in alcohols or phenols is known as acetylation. Acetylation of salicylic acid
produces aspirin.
Electrophilic aromatic substitution
In phenols, the
reactions that take place on the aromatic ring are electrophilic substitution
reactions. The –OH group attached to the benzene ring activates it towards
electrophilic substitution. Also, it directs the incoming group to ortho and
para positions in the ring as these positions become electron rich due to the
resonance effect caused by –OH group. The resonance structures are shown under
acidity of phenols.
Common electrophilic aromatic substitution reactions taking place in phenol are
as follows:
(3) Nitration:
With dilute nitric acid at
low temperature (298 K), phenol yields a mixture of ortho and para
nitrophenols.
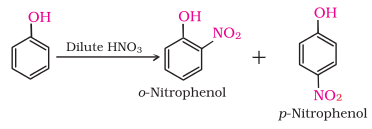
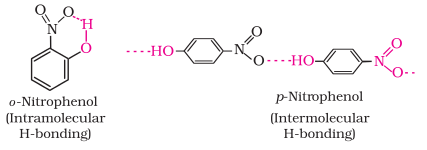
The ortho and
para isomers can be separated by steam distillation. o-Nitrophenol is steam volatile
due to intramolecular hydrogen bonding while p-nitrophenol is less volatile due
to intermolecular hydrogen bonding which causes the association of molecules.
With
concentrated nitric acid, phenol is converted to 2,4,6-trinitrophenol. The product
is commonly known as picric acid. The yield of the reaction product is poor.
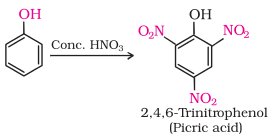
Nowadays picric
acid is prepared by treating phenol first with concentrated sulphuric acid
which converts it to phenol-2,4-disulphonic acid, and then with concentrated
nitric acid to get 2,4,6-trinitrophenol.
(4)
Halogenation:
On treating phenol with bromine, different reaction products
are formed under different experimental conditions.
(a) When the reaction is carried out in solvents of
low polarity such as CHCl3 or CS2 and at low
temperature, monobromophenols are formed.
The usual halogenation of benzene takes place in the
presence of a Lewis acid, such as FeBr3 , which polarises the
halogen molecule. In case of phenol, the polarisation of bromine molecule takes
place even in the absence of Lewis acid. It is due to the highly activating
effect of –OH group attached to the benzene ring.

(b) When
phenol is treated with bromine water, 2,4,6-tribromophenol is formed as white
precipitate.
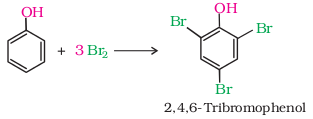
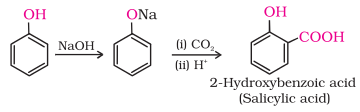
5. Kolbe’s
reaction
Phenoxide
ion generated by treating phenol with sodium hydroxide is even more reactive
than phenol towards electrophilic aromatic substitution. Hence, it undergoes electrophilic
substitution with carbon dioxide, a weak electrophile. Ortho hydroxybenzoic
acid is formed as the main reaction product.
6.
Reimer-Tiemann reaction
On
treating phenol with chloroform in the presence of sodium hydroxide, a –CHO
group is introduced at ortho position of benzene ring. This reaction is known as
Reimer – Tiemann reaction.
The intermediate substituted benzal chloride is hydrolysed in the presence of
alkali to produce salicylaldehyde

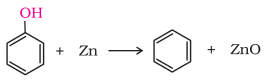
7. Phenol
with zinc dust
Phenol
is converted to benzene on heating with zinc dust.
8. Oxidation
Oxidation
of phenol with chromic acid produces a conjugated diketone known as
benzoquinone. In the presence of air, phenols are slowly oxidised to dark
coloured mixtures containing quinones.

Coupling
reactions
Phenol is dissolved
in sodium hydroxide solution to give a solution of sodium phenoxide.The
solution is cooled in ice, and cold benzenediazonium chloride solution is
added. There is a reaction between the diazonium ion and the phenoxide ion and
a yellow-orange solution or precipitate is formed. The product is one of the
simplest of what are known as azo compounds..
![]()
![]()
The naphthalen-2-ol
is dissolved in sodium hydroxide solution to produce an ion just like the
phenol one. This solution is cooled and mixed with the benzenediazonium
chloride solution. An intense orange-red precipitate is formed - another azo
compound.
![]()
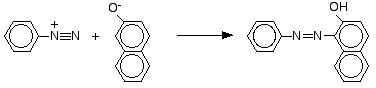
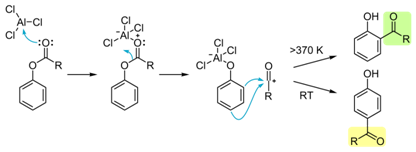
The Fries rearrangement
The Fries rearrangement, is a rearrangement reaction
of a phenolic ester to a hydroxy aryl ketone by catalysis of Lewis acids.It involves migration of an acyl
group of phenol ester to the aryl ring. The reaction is ortho and para
selective and one of the two products can be favoured by changing reaction
conditions, such as temperature and solvent.
The reaction progress is not dependent on solvent or
substrate. A widely accepted mechanism involves a carbocation intermediate.
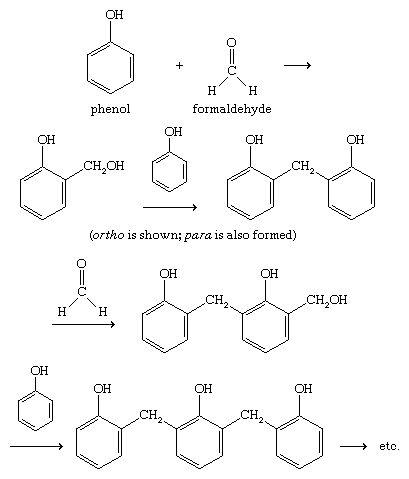
Phenol-formaldehyde resins
Phenol-formaldehyde resins , trade name Bakelite are inexpensive, heat-resistant, and waterproof, though somewhat brittle. The polymerization of phenol with formaldehyde involves electrophilic aromatic substitution at the ortho and para positions of phenol (probably somewhat randomly), followed by cross-linking of the polymeric chains.
Learning Chart of Phenol