1. Acetoacetic Ester Synthesis:- It is base-catalyzed alkylation or arylation of β-ketoesters. The products on mild hydrolysis and decarboxylation gives substituted acetones
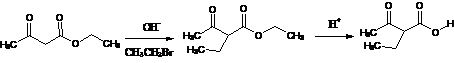
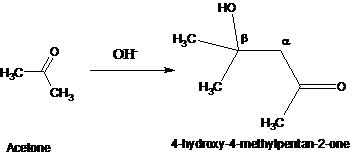
2. Aldol Reaction : Aldehyde or ketone with a hydrogen gives b hydroxyl aldehyde or ketone ( commonly called Aldol.) Traditionally, it is the acid- or base-catalyzed condensation of one carbonyl compound with the enolate/enol of another, which may or may not be the same, to generate a β-hydroxy carbonyl compound—an aldol.

3. Baekeland(Bakelite) Process:-Phenol reacts with formaldehyde in basic medium to form polymeric phenol-formaldehyde resin step by step.
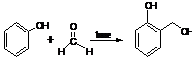
4. Baeyer-Villiger Oxidation:- Ketones are oxidized to esters , cyclic ketones to lactones and aldehydes to acids with Peroxy acids as CH3COOOH.
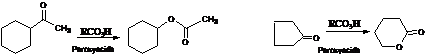
5. Beckmann Rearrangement; In acidic medium Oximes uudergoes rearrangement to form isomeric amides. Oximes of cyclic ketones give ring enlargements:
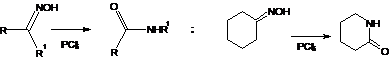
6. Benzilic
Acid Rearrangement (Benzil-Benzilic Acid Rearrangement):-Base-catalysed
rearrangement of
benzil to benzylic acid via phenyl group migration . It is similar
to intramolecular Cannizzaro reaction. 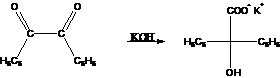
7.
Benzoin Condensation:-Aromatic aldehydes
(Benzaldehyde) give benzoins with KCN solution. 
8. Birch Reduction:-Aromatic Benzene rings get reduced at 1,4 position to form 1,4 -dihydrobenzene with alkali metals in liquid ammonia:
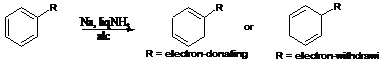
9. Blanc Reaction-(Effect of heat on dicarboxylic acids):-Dicarboxylic acids on heating with acetic anhydride gives either cyclic anhydrides or ketones depending on the respective positions of the carboxyl groups. 1,4- and 1,5-diacids yield anhydrides, while diacids in which the carboxy groups are in 1,6 positions yield ketones. (Note :- 1,2 or 1,3 dicarboxylic acids dissociate to form CO+CO2 and acetic acid respectively.)
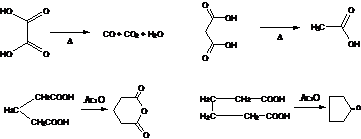
10. Cannizzaro Reaction:-It is base-catalyzed disproportionation reaction of aromatic or aliphatic aldehydes with out α-hydrogen to corresponding acid and alcohol. If the aldehydes are different, the reaction is called the “crossed Cannizarro reaction”:In such a case formaldehyde , if present , will always oxidize.
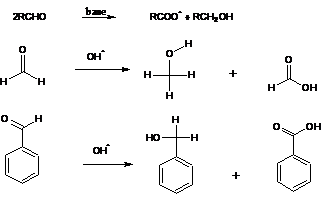
11. Claisen Condensation (Acetoacetic Ester Condensation):-Base-catalyzed condensation of an ester containing an α-hydrogen atom with a molecule of the same ester or a different one to give β-keto esters:
![]()
12. Claisen-Schmidt Condensation:-Condensation of an aromatic aldehyde with an aliphatic aldehyde or ketone in the presence of a relatively strong base (hydroxide or alkoxide ion) to form an α,β-unsaturated aldehyde or ketone:

13. Clemmensen Reduction:-Reduction of carbonyl groups of aldehydes and ketones to methylene groups with zinc amalgam and hydrochloric acid:
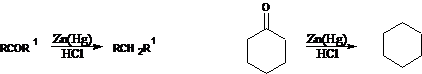
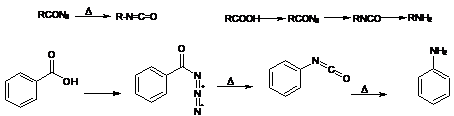
14. Curtius Rearrangement :-AcidAzides on by thermal decomposition gives isocyanates: The isocyanates on hydrolysis gives primary amines with one lesser carbin. The stepwise conversion of a carboxylic acid to an amine having one fewer carbon unit, via the azide and isocyanate, is referred to as the Curtius reaction:
15. Dieckmann Reaction:-Base-catalyzed cyclization of dicarboxylic acid esters to give β-ketoesters. It is also defined as intramolecular equivalent of the Claisen condensation,
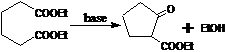
16. Dienone-Phenol Rearrangement:-Rearrangement of a 4,4-disubstituted cyclohexadienone into a 3,4-disubstituted phenol upon acid treatment:
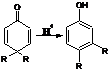
17. Etard Reaction;-Partial Oxidation of benzylic carbon with chromyl chloride to Benzaldehyde.
![]()
18. Fenton Reaction:-Oxidation of α-hydroxy acids with hydrogen peroxide and ferrous salts (Fenton's reagent) to α-keto acids or of 1,2-glycols to hydroxy aldehydes:
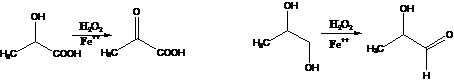
19. Fischer Osazone Reaction:-Formation of osazones by heating sugars with phenylhydrazine in dilute acetic acid: Osazones are a class of carbohydrate derivatives found in organic chemistry formed when sugars are reacted with phenylhydrazine
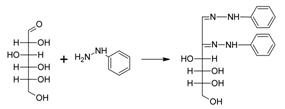
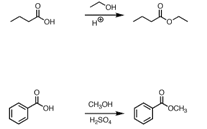
20. Fischer Esterification Method- Carboxylic acids form esters excess alcohol in the presence of hydrogen chloride or other acid catalysts:
21. Friedel-Crafts Alkylation Reaction:-The alkylation of benzene ring or aromatic compounds, catalyzed by aluminum chloride or other Lewis acids is called Friedel Craft reaction.:

22. Friedel-Crafts Acylation Reaction:-The acylation of benzene ring or aromatic compounds catalyzed by aluminum chloride or other Lewis acids is called friedel craft acylation:
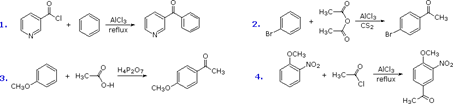
23. Fries Rearrangement-Rearrangement of phenolic esters to o- and/or p-phenolic ketones with Lewis acid catalysts
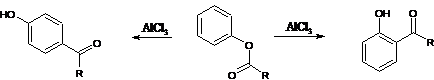
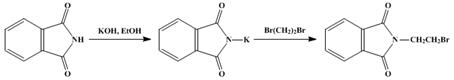
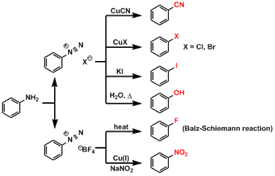
24. Gabriel Synthesis-Conversion of alkyl halides to primary amines by treatment with potassium phthalimide and subsequent hydrolysis
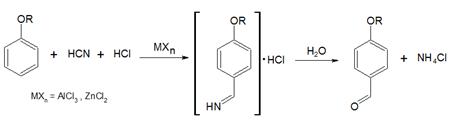
25. Gattermann Aldehyde Synthesis:-Preparation of phenolic aldehydes, phenol ethers or heterocyclic compounds by treatment of the benzene or aromatic substrate with hydrogen cyanide and hydrogen chloride in the presence of Lewis acid catalysts:
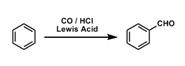
26. Gatterman-Koch Reaction: Formylation of aromatic hydrocarbons with CO and HCl and in the presence of Lewis' acids. The reaction also takes place with condensed polycyclic hydrocarbons, but phenoles do not react.
![]()
27. Griess Diazo Reaction;-Formation of aromatic diazonium salts from primary aromatic amines and NaNO2+HCl or nitrous acid at ice cold temperature of 0-50 C.
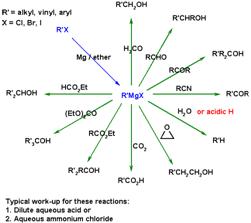
28. Grignard Reaction:-Traditionally, it is the addition of organomagnesium compounds (Grignard reagents) to carbonyl compounds to form alcohols.
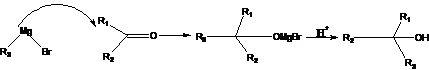
29. Harries Ozonide Reaction (Ozonolysis):-Treatment of olefins /alkenes with ozone form ozonoid. ozonoid hydrolysis yields two molecules of carbonyl compounds with Zn /H2O:
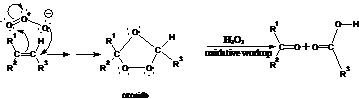
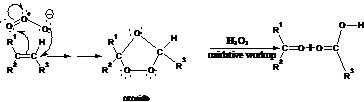
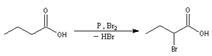
30. Hell-Volhard-Zelinsky Reaction-α-Halogenation of carboxylic acids in the presence of catalytic phosphorus is called HVZ reaction.:
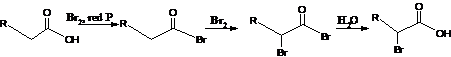
31.
Hofmann Degradation (Exhaustive Methylation)- Hofmann elimination, also known as exhaustive
methylation, is a process where an amine is reacted to create a tertiary amine and an alkene by treatment with excess methyl iodide followed by treatment with silver oxide, water, and heat. 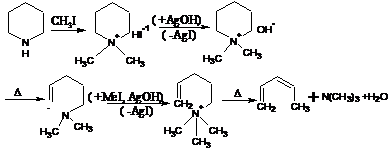
32. Hofmann Isonitrile Synthesis (Carbylamine Reaction)- Primary amines reacts with chloroform in the presence of alkali to form isonitriles or Carbylamines. The odor of the isocyanide is a test for a primary amine:
![]()
33. Hofmann Reaction-Conversion of primary carboxylic amides to primary amines with one fewer carbon atom upon treatment with hypohalites or hydroxide via the intermediate isocyanate:
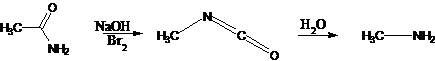
34. Hunsdiecker Reaction (Borodine Reaction)- Silver salts on thermal decarboxylation in the presence of halogens gives organic halides.
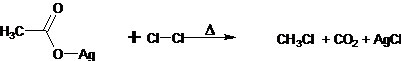
35. Hydroboration Reaction-Addition of boron hydrides to alkenes, allenes, and alkynes to form organoboranes, such that boron adds to the less substituted carbon. Attack usually takes place on the less hindered side in a cis fashion:
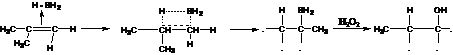
Oxidation of the hydroborated product gives anti markownikov addition product of water.
36. Jones Oxidation:-The oxidation of primary and secondary alcohols to acids and ketones, respectively, in the presence of chromic acid, aqueous sulfuric acid, and acetone. Isolated multiple bonds are not disturbed under these conditions:
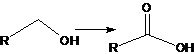
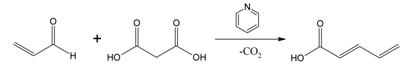
37. Knoevenagel Condensation : A Knoevenagel condensation is a nucleophilic addition of an active hydrogen compound to a carbonyl group followed by a dehydration reaction in which a molecule of water is eliminated (hence condensation).
The product is often an alpha, beta conjugated enone. In this reaction the carbonyl group is an aldehyde or a ketone. The catalyst is usually a weakly basic amine. The active hydrogen component has the form
Z–CH2-Z or Z–CHR–Z for instance diethyl malonate, ethyl acetoacetate or malonic acid
where Z is an electron withdrawing functional group. Z must be powerful enough to facilitate deprotonation to the enolate ion even with a mild base. Using a strong base in this reaction would induce self-condensationof the aldehyde or ketone.
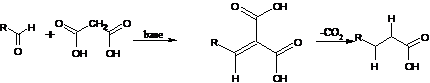 Kolbe Electrolytic Synthesis;-Formation
of symmetrical dimers by the electrolysis of carboxylates (decarboxylative
dimerization). The coupling of two distinct carboxylates yields unsymmetrical
products:
Kolbe Electrolytic Synthesis;-Formation
of symmetrical dimers by the electrolysis of carboxylates (decarboxylative
dimerization). The coupling of two distinct carboxylates yields unsymmetrical
products:

Kolbe-Schmitt Reaction:-Formation of aromatic hydroxy acids by carboxylation of phenolates, mostly in the ortho position, by carbon dioxide:
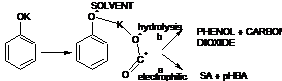
37. Lieben Iodoform Reaction (Haloform Reaction) – Aldehyde /Ketones with methylketo group gives yellow precipitate of iodoform with I2 in basic medium.
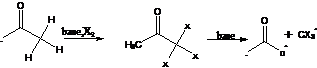
38. Lossen Rearrangement-Conversion of a hydroxamic acid to an isocyanate via the intermediacy of its O-acyl, sulfonyl, or phosphoryl derivative. In the presence of amines, ureas are formed; in the presence of water, amines containing one less carbon than the starting material, are generated:
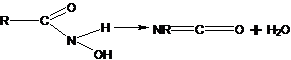
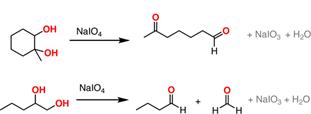
39. Malaprade Reaction (Periodic Acid Oxidation)-Compounds containing two hydroxyl groups, or a hydroxyl and an amino group, attached to adjacent carbon atoms, undergo cleavage of the carbon-carbon bond when treated with periodic acid to yield aldehydes:
40. Malonic Ester Syntheses-Syntheses based on the strongly activated methylene group of malonic esters which on reaction with sodium ethoxide form a resonance-stabilized ion that can be alkylated or acylated. After hydrolysis, the free alkylmalonic acids readily decarboxylate to mono- or disubstituted monocarboxylic acids:
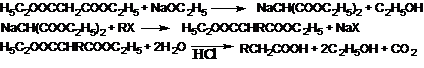
41. Meerwein-Ponndorf-Verley Reduction (Aluminum Alkoxide Reduction):- Reduction of aldehydes or ketones to the corresponding alcohols with aluminum alkoxides (the reverse of the Oppenauer oxidation,.):
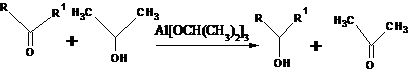
42. Meyer Synthesis (Victor Meyer Synthesis):- Formation of aliphatic nitrites and nitro derivatives by the reaction of aliphatic halides with metal nitrites:
![]()
43. Pinacol Coupling Reaction-Formation of pinacols by a reductive radical-radical coupling of carbonyl compounds, especially ketones:
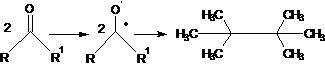
44. Pinacol Rearrangement-Acid-catalyzed rearrangement of vicinal diols to aldehydes or ketones:
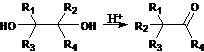
45. Reformatsky (Reformatskii) Reaction-Condensation of aldehydes or ketones with organozinc derivatives of α-halo esters to yield β-hydroxy esters:
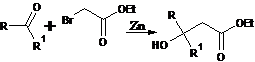
46. Reimer-Tiemann Reaction-Formation of phenolic aldehydes from phenols, chloroform and alkali:
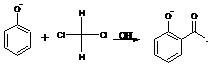
47. Rosenmund Reduction-Catalytic reduction of acid chlorides to aldehydes. To prevent further hydrogenation a poison is added to the catalyst:
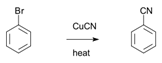
48. Rosenmund Synthesis-Conversion of aryl halides to aromatic nitriles in the presence of cuprous cyanide:
49. Sabatier-Senderens Reduction-Catalytic hydrogenation of organic compounds in the vapor phase by passage over hot, finely divided nickel (the oldest of all hydrogenation methods).
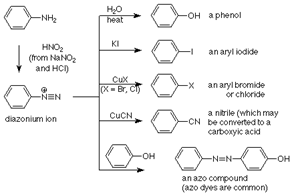
50. Sandmeyer Reaction;-Substitution of diazonium groups in aromatic compounds by halo or cyano groups in the presence of cuprous salts (Sandmeyer reaction), copper powder and hydrochloric or hydrobromic acid (Gattermann reaction) or cupric salts (Körner-Contardi reaction):

51. Sarett Oxidation; -Oxidation of primary and secondary alcohols to aldehydes (and/or carboxylic acids) and ketones by means of CrO3-pyridine complex:
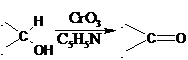
52. Schmidt Reaction-Acid-catalyzed addition of hydrazoic acid to carboxylic acids, aldehydes and ketones to give amines, nitriles and amides, respectively. Tertiary alcohols and substituted alkenes yield imines upon treatment with hydrazoic acid:
RCOOH
+ HN3 ![]() RNH2
+ CO2 + N2
RNH2
+ CO2 + N2
RCHO + HN3 ![]() RCN + N2 + H2O
RCN + N2 + H2O
RCOC + HN3 ![]() RCONHR + N2
RCONHR + N2
53. Schotten-Baumann Reaction-Acylation of alcohols or amines with acid chlorides in aqueous alkaline solution
ROH
+ C6H5COCl + NaOH ![]() C6H5COOR + NaCl +
H2O
C6H5COOR + NaCl +
H2O
54. Stephen Aldehyde Synthesis:-Reaction sequence employed to convert nitriles to aldehydes. Treatment of the nitrile with a mixture of stannous chloride and hydrochloric acid yields the imine salt complex which is subsequently hydrolyzed to the aldehyde. Practically applied only to aromatic aldehydes:
![]()
55. Swarts Reaction:-Fluorination of organic polyhalides with antimony trifluoride (or zinc and mercury fluorides) in the presence of a trace of a pentavalent antimony salt:
![]()
56. Tiffeneau-Demjanov Rearrangement:-Rearrangement of β-amino alcohols upon diazotization with nitrous acid to give carbonyl compounds. Cyclic alcohols yield ring expanded or contracted products:
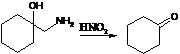
57. Tishchenko Reaction:-Formation of esters from aldehydes by an oxidation-reduction process in the presence of aluminum or sodium alkoxides:
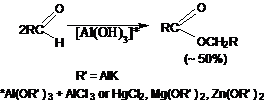
58. Ullmann Reaction:-Copper-mediated coupling of aryl halides. Biaryl ether synthesis is similarly accomplished with aryl halides and phenols:
![]()
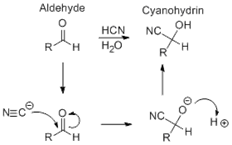
59. Urech Cyanohydrin Method; Cyanohydrin formation by addition of alkali cyanide to the carbonyl group in the presence of acetic acid (Urech method) or by reaction of the carbonyl compound with anhydrous hydrogen cyanide in the presence of a basic catalyst (Ultee cyanohydrin method):
60. Wacker Oxidation:-The oxidation of ethylene to acetaldehyde employing palladium chloride and cupric chloride as catalysts and molecular oxygen as oxidant. The reaction has been extensively developed for the oxidation of terminal alkenes to methyl ketones:
![]()
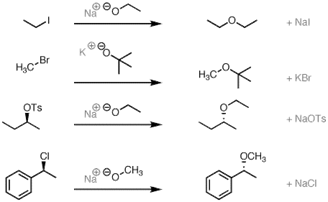
61. Williamson Synthesis:_Synthesis of ethers by alkylation of alkoxides with alkyl halides or alkyl sulfates:
![]()
62. Wittig Reaction; Horner Reaction; :-Alkene formation from carbonyl compounds and phosphonium ylides, proceeding primarily through the proposed betaine and/or oxaphosphetane intermediates. The stereoselectivity can be controlled by the choice of ylide, carbonyl compound, and reaction conditions:

63. Wolff-Kishner Reduction; Complete reduction of carbonyl compounds to methyl or methylene groups on heating with hydrazine hydrate and a base. In the Huang-Minlon modification diethylene glycol is used as a solvent:

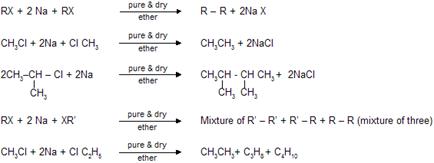
64. Wurtz-Fittig Reaction-Formation of alkylated aromatic hydrocarbons on coupling of an alkyl and an aryl halide with sodium: