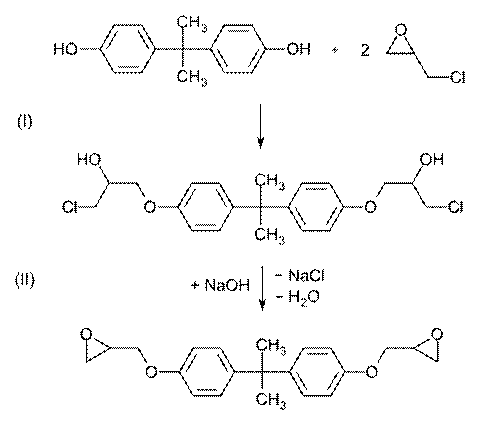
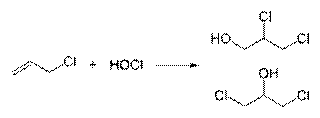
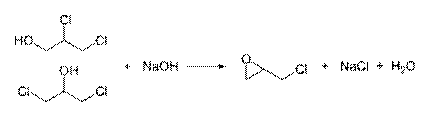
Epoxides
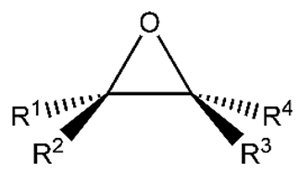
Epoxides : a class of cyclic ether compounds with three-atom rings Strained ,more highly reactive than other ethers.
Epoxides are electrophilic due to strained three-membered ring system; nucleophilic attack at carbon releases the ring strain.
A compound containing the epoxide functional group can be called an epoxy, epoxide, oxirane, and alkeneoxide.
Epoxides are In general, low molecular weight ,colourless and nonpolar, and often volatile.
Synthesis
alkenes with peroxycarboxylic acids:
Direct oxidation of alkenes with peroxycarboxylic . (PBA or mcpba)
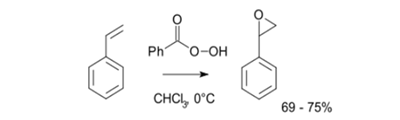
(Note: Oxidation of alkene with performic or peracetic acid gives diol.)
Olefin oxidation using Ag2O :
Oxidation
of ethene with oxygen over a silver oxide catalyst at 300o:
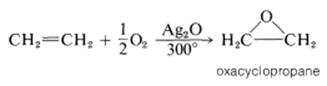
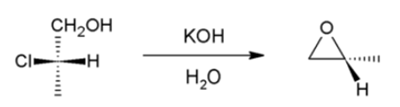
Chlorohydrins to Epoxide :
Intramolecular SN2 substitution: In this case, an alkoxide ion intramolecularly displaces chloride.
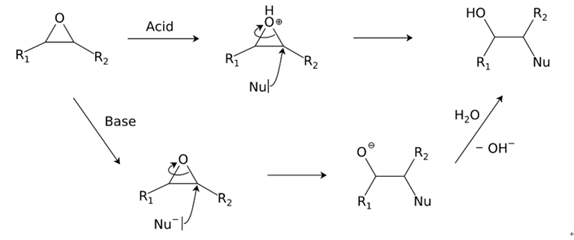
Reactions
Ring-opening reactions dominate the reactivity of epoxides, as they are potent electrophiles.Alcohols, water, amines, thiols and many other reagents can act as nucleophile for this reaction.
This reaction is the basis of the formation of epoxy glues and the
production of glycols.
Applications