Electrophilicity is defined as the relative reactivity of an electrophilic reagent.
However, whereas Lewis acidity is measured by relative equilibrium constants, electrophilicity is measured by relative rate constants for reactions of different electrophilic reagents towards a common substrate (usually involving attack at a carbon atom).
Relative Electrophilicity in Carbonyl and Carboxylic acid derivatives,
Carboxylic acid derivatives react tend to react via nucleophilic acyl substitution where the group on the acyl unit, R-C=O undergoes substitution:
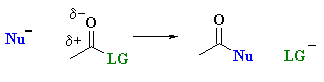
The observed relative reactivity order is shown below:
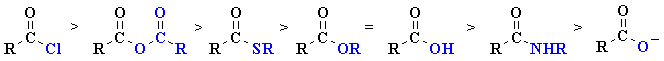
|
It
is useful to view the carboxylic acid derivatives as an acyl
group, R-C=O, with a
different substituent attached.
|
Carbonyls as aldehydes and Ketones Carbon atoms are less electrophilic than Acid Chloride and acid anhydrides but more electrophilic than thioester, ester, acid amide and Carboxylates.