Synthesis of carboxylic acids
1. Oxidation of primary alcohols with chromic acid or KMnO4
2. Cleavage of alkenes with vigorous KMnO4:This gives ketones or carboxylic acids, depending on the specific
alkene.
3. Cleavage of alkynes with O3 or vigorous KMnO4
4. Carboxylation of Grignards
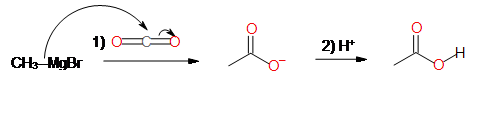
Reactions of Carboxylic Acids
1.
Hydrolysis of acid derivatives

2.
Fischer Esterification
Overall: Carboxylic acid + alcohol → ester +
water
Step 1: protonation of
carbonyl to “activate” it for attack.

Step 2: Alcohol attacks
carbonyl carbon of the acid

Step 3: Deprotonation of
the oxygen from the alcohol
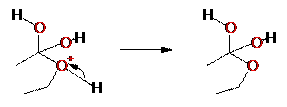
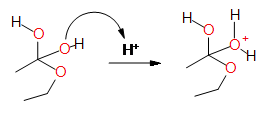
Step 4: Protonation of one
of the oxygens from the acid
Step 5: Loss of water,
forming a resonance-stabilized carbocation
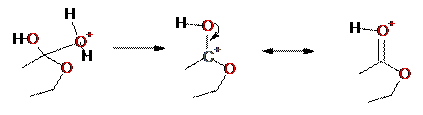
Step 6: Deprotonation of
carbonyl

3.
Hydrolysis of esters: When it’s base-catalyzed, it’s called
saponification.
4.
Hydrolyzing triglycerides with base, gives carboxylates
with long fatty chains.
These chains form micelles. Water-soluble with lipophilic interiors.

5.
Direct formation of amides: Acid on strong heating with amine
gives amide.
Step 1: Acid Base reaction

On strong heating amide is
formed.
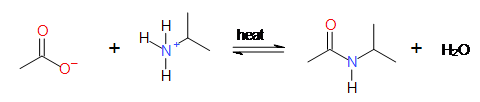
6.
Polymerization: Dicarboxylic acids
with difunctional groups form long chains

7.
Reaction with alkyl lithiums: The alkyl
lithium first deprotonates the carboxyl group, giving the carboxylate.A
second alkyl lithium then adds to the carbonyl, which after protonation, forms the ketone.
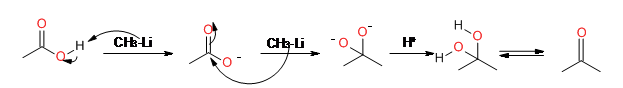
8.
Reduction of carboxylic acids
1.
COOH to 1° alcohol with LiAlH4