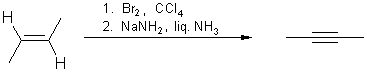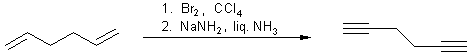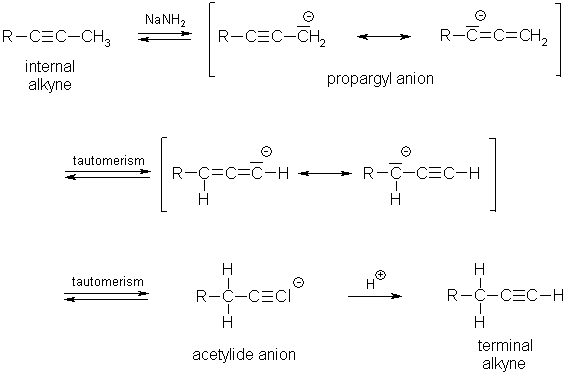Alkynes: Preparation
1. Calcium carbide
with water:
The reaction of calcium carbide with water is carried out at room
temperature and, for a long time,
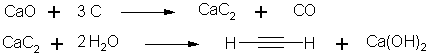
2. Pyrolysis of methane:
At 1500 °C, methane is pyrolyzed using short reaction times. The reaction is endothermic, however, at very high temperatures it becomes
exothermic
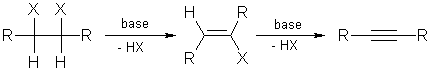
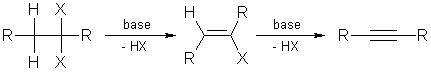
3. Elimination of 1,1- or 1,2-dihalogen alkanes:
Double elimination of 1,1- or 1,2-dihalogen alkanes with strong bases
yields the corresponding compounds with triple bonds. Elimination with sodium
amide in ammonia takes place at ( -33 °C).
Dihalogen alkanes are easily obtained from alkenes by halogenation and
these compounds can be transferred into alkynes by double dehydrohalogenation.
4.
Terminal Alkyne to Internal Alkyne:
Acetylide anion is very
nucleophilic and reacts with a multitude of electrophiles in SN2-type reactions. Using this method, terminal and internal alkynes can be
synthesized.
Alkynes: Reactions
Alkynes
are very reactive compounds and the triple bond participates in many
electrophilic addition reactions.
1. Combustion of ethyne:
![]()
Acetylene is frequently used for welding purposes. Mixtures of ethyne
with oxygen are explosive over a wide range of composition (1.5 and 82 Vol % ).
2. Hydrogenation of ethyne:
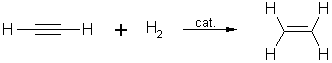
During
the catalytic hydrogenation of ethyne,
ethene is formed first which in the next step is further reduced to ethane.
In this
reaction, the heat of hydrogenation of the first π bond is higher than
that of the second.
Internal
alkynes are more stable than terminal ones.
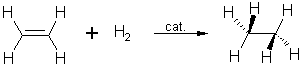
Heat of hydrogenation
|
Ethyne to ethene |
ΔH° = - 175.4 kJmol-1 |
|
Ethene to ethane |
ΔH° = - 136.9 kJmol-1 |
|
But-1-yne to butane |
ΔH° = - 292.7 kJmol-1 |
|
But-2-yne to butane |
ΔH° = - 272.6 kJmol-1 |
3. Partial Hydrogenation of Alkyne:
By using a less active (partially poisoned) catalyst, hydrogenation can be stopped at the alkene stage.
Lindlar catalyst (palladium
on CaCO3,
poisoned with quinoline) is frequently used for this hydrogenation which
stereospecifically yields cis products.
Rosenmund catalyst (palladium
on BaSO4)
is also used for the same purpose and yields cis products.
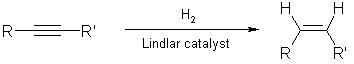
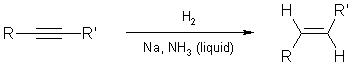
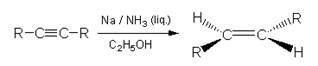
4. Reduction of alkynes:
with
sodium in liquid ammonia (solvated electrons) yields trans alkenes.
5. Addition of hydrogen halides to alkynes:
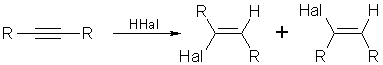
EAR:
The mechanism involves protonation of the triple bond to form an
alkenyl cation which subsequently is captured by a counter ion. It is difficult
to limit the addition to only one HX molecule because the resulting double bond
normally is more reactive than than the alkyne.
6. Halogenation of alkynes :
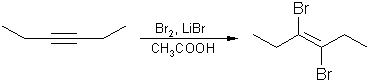
The electrophilic
addition of halogens to alkynes to yield tetrahaolgen alkanes
proceeds via vicinal dihalogen
alkenes as intermediates. As a rule, the addition normally gives
the trans product.
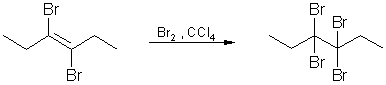
7.
Hydration of alkynes:
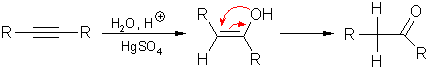
Catalyzed
by mercury(II) salts, water can be added to alkynes according to the
Markovnikov rule.
This
reaction yields enoles which tautomerize to the corresponding
carbonyl compounds.
Ethyne
yields acetaldehyde;
Terminal
alkynes produce methyl ketones.
8. (Hydroboration-Oxidation:)
Overall
antimarkonikov syn addition of water takes place.
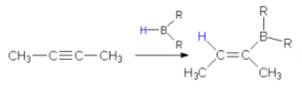
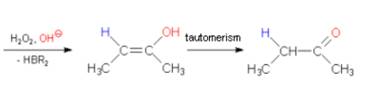
This
reaction yields enoles which tautomerize to the corresponding
carbonyl compounds.
Ethyne
yields acetaldehyde;
Terminal
alkynes produce aldehydes.
Internal
alkynes produce Ketones.
9. Nucleophilic addition to alkynes:
Alkynes
also undergo nucleophilic addition reaction but only under harsh conditions in
presence of strong electron withdrawing groups.
10. Polymerization of ethyne:
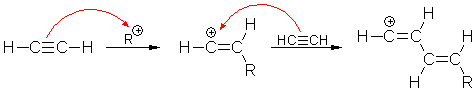
Polymerization of is initiated by carbenium ions. Subsequent chain
reaction yields a long-chain molecule containing conjugated double bonds
11.
Alkynes: Isomerization:
Since higher-substituted alkyl alkynes (internal alkynes) are
more stable than terminal alkynes
(hyperconjugation), isomerization is favored thermodynamically.
The deciding step is the tautomerization of the
acetylide anion to the propargyl anion which is stabilized by mesomerism.
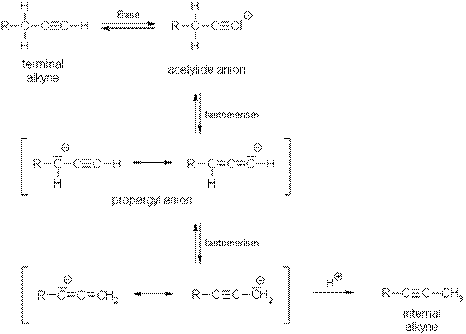
The
triple bond migrates from the terminal position into the C-C chain.
Isomerization in the opposite direction leading to the formation
of a terminal alkyne can be accomplished with strong
bases, e.g. sodium amide at 150 °C, which
are able to completely deprotonate terminal alkynes.
The reaction
proceeds in the opposite direction because the most stable anion (acetylide) is
formed under the strong basic conditions and not the more stable hydrocarbon
(internal alkyne) which is formed under less basic conditions.