Reactions of Alkene
By S.K.Sinha (Resonance, Kota)
Alkenes are hydrocarbons that contain one or more double bonds. The π-electrons also make the carbons atom electron-rich and attract an electrophile. Thus, alkenes can undergo a large variety of reactions. Alkenes can be used to synthesize a wide variety of compounds, such as halide, alcohol, ethers, and alkanes.
1. Hydrogenation :
Hydrogenation is the addition of hydrogen (H2) to the π-bond of alkenes, producing an alkane. The reaction must be catalyzed by metals such as Pd, Pt, Rh, and Ni. Catalytic on metal surface.
It is example of SYN Addition.
Example
1. Ethene (C2H4) converts into ethane (C2H6) by reacting with hydrogen at 150 ͦC.
C2H4 + H2 → C2H6
2. Cyclohexene (C6H10) reacts with hydrogen in the presence of palladium (Pd) catalyst to produce cyclohexane (C6H12).
C6H10 + H2 → C6H12
2. Halogenation
Halogenation of alkene is an addition reaction where a halide, such as Cl2 or Br2, gets attached to the alkene breaking the double bond between the carbon atoms. The resulting product is a vicinal (neighboring) dihalide.
Example
1. Ethene (CH2 = CH2) reacts with chlorine (Cl2) in the presence of carbon tetrachloride (CCl4), producing dichloroethane (CH2Cl – ClCH2).
CH2 = CH2 + Cl2 → CH2Cl – ClCH2
2. Ethene (CH2 = CH2) reacts with bromine (Br2) in the presence of carbon tetrachloride (CCl4), producing dibromoethane (CH2Br –CH2Br).
CH2 = CH2 + Br2 → CH2Br –CH2Br
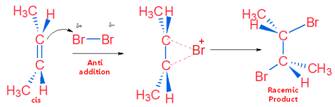
It is example of Anti Addition. The reaction proceeds with cyclic bromonium (non-classical) ion.
Anti addition of Br2 on Cis Compound
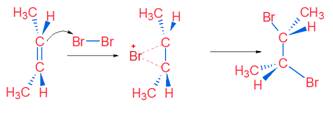
Anti addition of Br2 on trans Compound
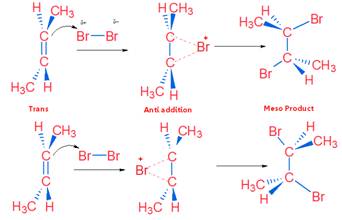
Tips: Cis compound on anti addition gives Racemic product (CAR) & Trans compound on anti addition gives Meso product (TRAM)
3. Hydrohalogenation:
In hydrohalogenation of alkenes, the double bond between carbon atom breaks, followed by the electrophilic addition of a hydrogen atom and halogen. Following Markovnikov’s rule, the halide gets attached to the more substituted carbon. The resultant product is a haloalkane or alkyl halide.
Examples
1. Ethene (CH2=CH2) reacts with HBr in the presence of CCl4, producing bromoethane or ethyl bromide (CH3CH2Br).
CH2=CH2 + HBr → CH3CH2Br
2. Propene (CH3CH=CH2) reacts with hydrogen chloride, producing 2-chloropropane or 2-propyl chloride (CH3CHClCH3) as the major product and 1-chloropropane (CH3CH2CH2Cl) as the minor product.
CH3CH=CH2 + HCl → CH3CHClCH3 (major product, more stable) + CH3CH2CH2Cl (minor product, less stable)
Here, the two products CH3CHClCH3 and CH3CH2CH2Cl are constitutional isomers.
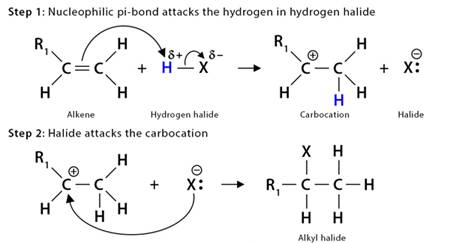
Mechanism of Alkene Reaction : Let us take the example of the hydrohalogenation reaction. It is an electrophilic addition reaction as an electrophile initially attacks the substrate.
The fundamental mechanism of hydrohalogenation of alkenes is as follows:
1. First, the nucleophilic π-bond of the alkene breaks down as it tries to grab the electrophilic H+ from hydrogen halides, such as HBr.
2. Then, following Markovnikov’s rule, H+ gets added to the less substituted carbon atom. The more substituted carbon is now deficient in electrons, thus turning into a carbocation.Greater the stability of Carbocation , faster the rate of reaction.
3. Finally, the halide ion (Br–) present in the solution attacks the carbocation, forming alkyl bromide.
4. Hydration
The addition of water to alkenes is known as hydration. In this reaction, the π-bond of alkene and OH bond in water breaks, producing alcohol. The hydration process usually makes many simple alcohols of alkenes in the presence of an acid catalyst.
Example
Ethene (CH2=CH2) reacts with water in the presence of a strong acid such as sulfuric acid (H2SO4) to produce ethanol (CH3CH2OH).
CH2=CH2 + H2O → CH3CH2OH
5. Polymerization
In this addition reaction, an alkene reacts with itself to form a high‐molecular‐weight compound composed of repeating units of the original compound. Here, the alkene acts as a monomer, producing polymer by joining its repeated units to give a single product.
Example
During the polymerization of ethene (CH2=CH2), thousands of ethene molecules join together to make poly(ethene) or polythene (-CH2-CH2-CH2– CH2– CH2-)n.
[CH2=CH2]n → (-CH2-CH2-CH2-CH2-CH2-)n
6. Oxidation :
Alkenes can easily undergo oxidation under the influence of oxidizing agents, such as potassium permanganate. The products formed depend on the reaction conditions. At low temperatures and low concentrations of oxidizing reagents, alkenes tend to form glycols.
Example
Ethene (CH2=CH2) reacts with 1-4 % potassium permanganate (KMnO4) solution to produce ethylene glycol (HOCH2-CH2OH), manganese dioxide (MnO2), and potassium hydroxide (KOH).
3C2H4 + 2KMnO4 + 4H2O → 3HOCH2-CH2OH + 2MnO2 + 2KOH
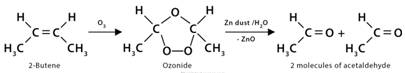
7. Ozonolysis
Ozonolysis is the oxidation of alkenes by ozone. Here, both the σ and π bonds of the alkenes get destructed. Ozone (O3) has a dipolar ion structure which first adds to the π-bond of alkene to form a highly unstable molozonide structure and then gets rearranged to another structure by breaking of C-C σ-bond to give ozonide. It is further cleaved under a reductive atmosphere using Zn dust/H2O to form corresponding carbonyl compounds, like aldehydes and ketones. This oxidative cleavage of an alkene double bond generally accomplishes good yield.
Example
1. Ozonolysis of 2-butene: Here, 2-butene (CH3CH=CHCH3) yields two acetaldehyde molecules (CH3CHO) after undergoing ozonolysis.
CH3CH=CHCH3 + O3 → Ozonide
Ozonide → 2CH3COH + H2O + ZnO
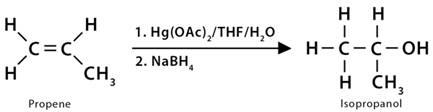
8. Oxymercuration
In this reaction, an alkene reacts with mercuric acetate [Hg(OAc)2] in the presence of an oxygen nucleophile, such as water or alcohol, to form an organomercury intermediate, such as hydroxyl or ethoxy mercurial compounds. The bond present between the carbon and mercury of the intermediate product gets converted to a carbon-hydrogen bond after treatment with sodium borohydride (NaBH4). As a result of the overall reaction, the alkene converts into alcohol or ether, depending on the nucleophile used.
If the nucleophile is water, the alkene gets converted into alcohol.
If the nucleophile is an alcohol, the alkene gets converted into an ether. Thus, the hydroxyl or ethoxy mercurial compounds are further reduced to alcohols or ethers, respectively.
Example
1. Propene (CH3CH=CH2) reacts with water and mercuric acetate (Hg(OAc)2) followed by NaBH4, giving isopropyl alcohol or 2-propanol (CH3CHOHCH3). The overall reaction is the electrophilic addition of water to the alkene following Markovnikov’s rule.
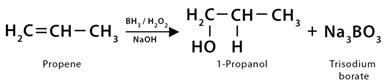
9. Hydroboration
Hydroboration is an oxidation reaction in which alkenes transform into alcohol by reacting with water. It is a two-step reaction. In the first step, diborane (B2H6) is added to form trialkyl boranes. The reaction proceeds in an anti-Markovnikov manner. The hydrogen from B2H6 attaches to the more substituted carbon, and the boron attaches to the least substituted carbon in the alkene double bond. The trialkyl borane is oxidized using an alkaline hydrogen peroxide solution in the next step. It leads to the formation of primary alcohols.
Example
1. In the hydroboration of propene (CH3CH=CH2), diborane (B2H6) is added in the first step to form trialkyl boranes [(CH3CH2CH2)3B]. The next step is the oxidation of trialkyl borane, which is done using an alkaline hydrogen peroxide (H2O2) solution, leading to the formation of 1-propanol (CH3CH2CH2OH) along with trisodium orthoborate (Na3BO3).
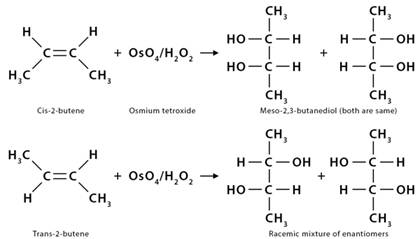
10. Dihydroxylation
Dihydroxylation is when an alkene is converted into a vicinal diol. The most common and direct process for this oxidation is using a high-oxidation-state transition metal, typically osmium or manganese. The metal is often used as a catalyst, with other oxidizing agents like hydrogen peroxide (H2O2).
Example
1. In the dihydroxylation of 2-butene (CH3CH=CHCH3), it reacts with osmium tetroxide (OsO4) in the presence of hydrogen peroxide (H2O2). The products depend on the type of butane used. If it is cis-2-butene, the products are meso-2,3-butanediol (CH3CH(OH)CH(OH)CH3. On the other hand, if it is trans-2-butene, the products are a racemic mixture of enantiomers.